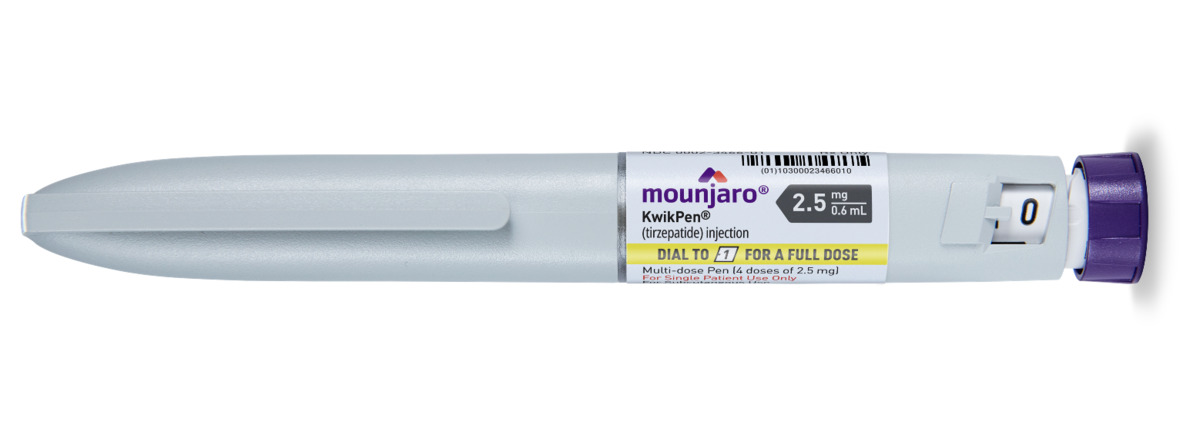
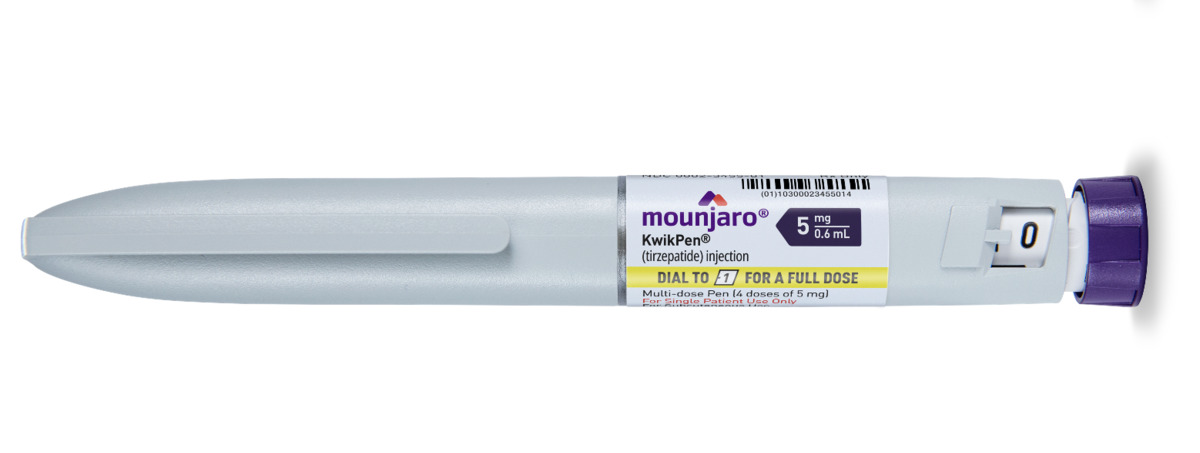
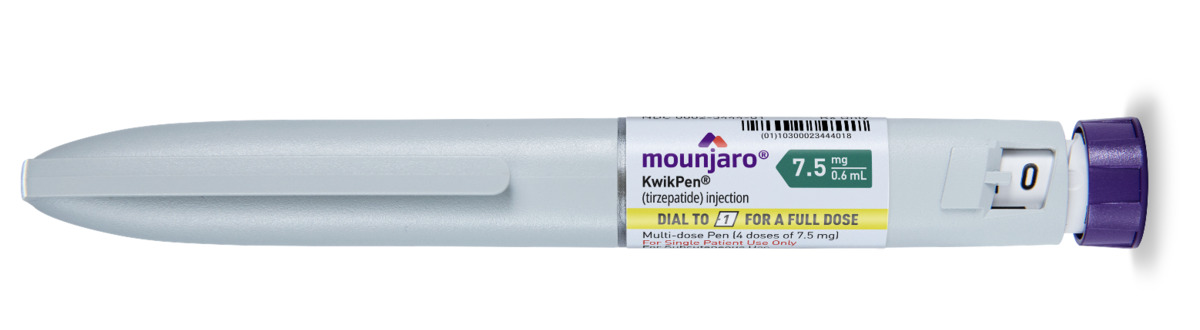
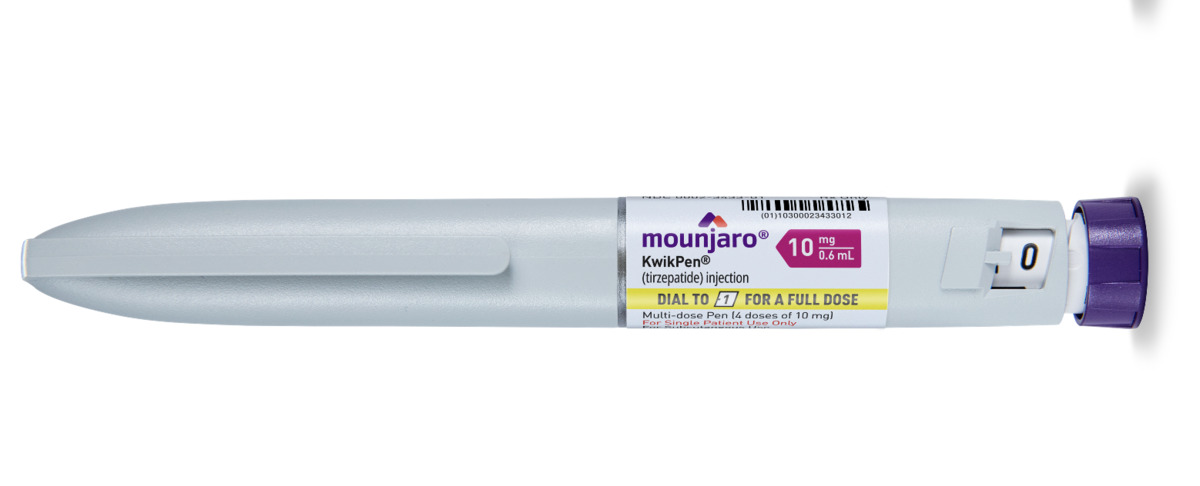
Other Options Include
Mounjaro is currently out of stock. Fresh stock is expected from 2nd September, with updated pricing from Eli Lilly.
In the meantime, we are pleased to confirm that Wegovy is in stock and available today. [Order Wegovy today and begin your treatment sooner]
Wegovy is a clinically proven, once-weekly treatment for weight management, designed to support effective and sustainable results. Many patients have already made the switch and are experiencing the benefits of starting treatment without delay.
What is Mounjaro (Tirzepatide) for Weight Loss?
Mounjaro® (Tirzepatide) is a once-weekly injectable medication developed by Eli Lilly. As of 2024, the MHRA (Medicines and Healthcare products Regulatory Agency) has authorised Mounjaro for use in adults with obesity (BMI ≥30) or overweight (BMI ≥27) with at least one weight-related comorbidity, such as hypertension, dyslipidaemia, or obstructive sleep apnoea. Mounjaro represents a significant advancement in the medical treatment of obesity, offering a new therapeutic option for individuals struggling to achieve and maintain a healthier weight.
Benefits of Losing Weight
Losing weight offers numerous health benefits that can improve both your quality of life and long-term wellbeing. Achieving and maintaining a healthy weight helps reduce the risk of chronic conditions such as type 2 diabetes, heart disease, high blood pressure, and stroke. Weight loss can also improve joint health, reducing pain and stiffness associated with arthritis. Additionally, shedding excess weight often leads to better sleep quality, increased energy levels, and enhanced mental health by lowering stress, anxiety, and depression. For many, weight loss boosts confidence and supports a more active lifestyle, enabling greater participation in everyday activities and hobbies. Overall, effective weight management is a key step toward living a healthier, longer life.
How Mounjaro Works for Weight Loss
Mounjaro is the first and only approved treatment in the UK that acts as a dual GIP and GLP-1 receptor agonist. It works by mimicking the actions of two key gut hormones:
- GIP (glucose-dependent insulinotropic polypeptide): Supports fat metabolism and insulin sensitivity.
- GLP-1 (glucagon-like peptide-1): Suppresses appetite, enhances satiety, and slows gastric emptying.
This dual hormone action helps reduce hunger, regulate calorie intake, and support sustained weight loss over time. Clinical trials have shown that Tirzepatide can lead to substantial and clinically meaningful reductions in body weight, especially when combined with a reduced-calorie diet and increased physical activity.
Is Mounjaro Right for You?
Mounjaro is available by prescription and should be used under the guidance of a qualified healthcare professional. It is typically considered when lifestyle interventions alone are insufficient to achieve weight loss goals. Treatment is most effective when combined with long-term lifestyle changes, including. dietary improvements and regular physical activity.
How is Mounjaro Taken?
Mounjaro (tirzepatide) is administered as a once-weekly subcutaneous injection, typically into the abdomen, thigh, or upper arm. The injection is delivered using a pre-filled, single-dose pen designed for easy at-home use. Patients can administer the injection themselves after receiving appropriate guidance from a healthcare provider. Mounjaro can be taken at any time of day, with or without food, and should be injected on the same day each week. This once-weekly injection makes Mounjaro a practical option for people managing busy schedules or new to injectable weight loss treatments.
Dosage and Titration Schedule
Mounjaro is started at a low initial dose to help minimise side effects, particularly gastrointestinal symptoms such as nausea. The typical starting dose is 2.5 mg once weekly for four weeks. After this period, the dose is gradually increased in 2.5 mg increments every four weeks, based on individual response and tolerability. The maximum recommended dose for weight loss is 15 mg once weekly. This titration schedule allows the body to adjust to the medication while maximising both safety and effectiveness. Your healthcare provider will closely monitor your progress and adjust the dosage accordingly.
What Does the Research Say About Mounjaro?
Mounjaro has been extensively studied in both diabetes and weight loss populations. In the SURMOUNT clinical trials, tirzepatide demonstrated superior weight loss results compared to other medications, including GLP-1 receptor agonists like semaglutide (Wegovy). Participants without diabetes lost an average of 15% to 22.5% of their body weight, depending on the dose. These results have positioned Mounjaro as a leading treatment for obesity, now licensed in the UK for this indication. Ongoing research continues to evaluate its long-term safety, metabolic benefits, and potential role in preventing obesity-related conditions such as type 2 diabetes, heart disease, and sleep apnoea.
Mounjaro and Contraception
The effectiveness of birth control pills may be reduced when starting Mounjaro (tirzepatide) or after any increase in your dose. To ensure full contraceptive protection, it is essential to use an additional barrier method of contraception, such as condoms, or switch to a non-oral contraceptive method like an intrauterine device (coil) during this time. You should continue using these additional or alternative contraception methods for at least four weeks after starting Mounjaro, and for four weeks following any dose escalation. Always discuss your contraception options with your healthcare provider before beginning treatment with Mounjaro.
Warnings
Mounjaro may cause tumours in the thyroid, including thyroid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath. If you have any of these symptoms, stop treatment straight away, inform us and see your healthcare provider immediately.
Do not use Mounjaro if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC).
Do not use Mounjaro if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Do not use Mounjaro if you are allergic to it or any of the ingredients in Mounjaro.
Mounjaro (Tirzepatide) and Mental Health: What You Should Know
While Mounjaro is primarily used for weight management, it's important to be aware of its potential effects on mental health. Some patients have reported mood changes, including symptoms of depression or anxiety, during treatment. If you have a history of depression or other mental health conditions, or if you notice new or worsening feelings of sadness, irritability, or changes in mood while using Mounjaro, it is crucial to get in touch and speak with a healthcare professional immediately. A clinician can help assess your symptoms and recommend appropriate support or treatment. Maintaining open communication about your mental health ensures the safest and most effective use of Mounjaro.
Mounjaro (Tirzepatide) and Pregnancy or Breastfeeding
Side Effects of Mounjaro
While Mounjaro (Tirzepatide) is an effective treatment for weight management, it is important to be aware of potential side effects, both common and serious. Common side effects may include nausea, diarrhoea, decreased appetite, vomiting, constipation, indigestion, and stomach pain. These symptoms are typically mild to moderate in intensity and often improve over time. To minimise discomfort, healthcare professionals recommend gradual dose escalation, also known as titration, which allows the body to adjust to the medication in a stepwise manner.
- Inflammation of the pancreas (pancreatitis). Stop using Mounjaro and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Mounjaro with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. Signs and symptoms of low blood sugar may include dizziness or light-headedness, sweating, confusion or drowsiness, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability, or mood changes, hunger, weakness and feeling jittery.
- Serious allergic reactions. Stop using Mounjaro and get medical help right away if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue or throat, problems breathing or swallowing, severe rash or itching, fainting or feeling dizzy, and very rapid heartbeat.
- Kidney problems (kidney failure). In people who have kidney problems, diarrhoea, nausea, and vomiting may cause a loss of fluids (dehydration), which may cause kidney problems to get worse. It is important for you to drink fluids to help reduce your chance of dehydration. Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Mounjaro. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
- Changes in vision. Tell your healthcare provider if you have changes in vision during treatment with Mounjaro.
- Gallbladder problems. Gallbladder problems have occurred in some people who use Mounjaro. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), and clay-colored stools.
Food or liquid getting into the lungs during surgery or other procedures that use anaesthesia or deep sleepiness (deep sedation). Mounjaro may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Mounjaro before you are scheduled to have surgery or other procedures.
Mounjaro Patient Information Leaflet (PIL)
For more information on Mounjaro, please see the PIL, available via the link below:
Patient counseling:
- Liraglutide must be taken with the recommended healthy balanced diet containing less than a regular exercise routine.
- Watch our online video to learn proper injection techniques
- tirzepatide should be injected at the same time every week and can be injected before or after food
- The dosage for this medication needs to be tapered up in order to avoid any gastro-intestinal side effects. Doses should be maintained for 1 week before being increased by 0.6mg every week until a dose of 3mg/day is reached. After that, the dose should be maintained at 3mg/day
- Injections should be issued in the upper thigh, upper arm, or abdomen around the umbilical region and rotate injection sites.
- If a dose is missed, it should be administered as soon as possible and within 5 days after the missed dose. If more than 5 days have passed, the missed dose should be skipped, and the next dose should be administered on the regularly scheduled day. In each case, patients can then resume their regular once-weekly dosing schedule. If more doses are missed, reducing the starting dose for re-initiation should be considered
- The day of weekly administration can be changed if necessary as long as the time between two doses is at least 3 days (>72 hours). After selecting a new dosing day, once-weekly dosing should be continued.
Special warnings and precautions for use
Gastrointestinal effects
The use of GIP/GLP-1 receptor agonists may be associated with gastrointestinal adverse reactions that can cause dehydration, which in rare cases can lead to a deterioration of renal function. Patients should be advised of the potential risk of dehydration in relation to gastrointestinal side effects and take precautions to avoid fluid depletion.
Acute pancreatitis
Acute pancreatitis has been observed with the use of GIP/GLP-1 receptor agonists. Patients should be informed of the characteristic symptoms of acute pancreatitis. If pancreatitis is suspected, tirzepatide should be discontinued; if confirmed, tirzepatide should not be restarted. Caution should be exercised in patients with a history of pancreatitis.
In the absence of other signs and symptoms of acute pancreatitis, elevations in pancreatic enzymes alone are not predictive of acute pancreatitis.
For patients with diabetes
Tirzepatide must not be used as a substitute for insulin in patients with diabetes.
Hypoglycaemia in patients with diabetes
Insulin and sulfonylurea are known to cause hypoglycaemia. Patients treated with tirzepatide in combination with a sulfonylurea or insulin may have an increased risk of hypoglycaemia. The risk of hypoglycaemia can be lowered by reducing the dose of sulfonylurea or insulin when initiating treatment with a GIP/GLP-1 receptor agonist. The addition of tirzepatide 2.4 mg in patients treated with insulin has not been evaluated.
Diabetic retinopathy in patients with type 2 diabetes
In patients with diabetic retinopathy treated with insulin and, an increased risk of developing diabetic retinopathy complications has been observed. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy, but other mechanisms cannot be excluded. Patients with diabetic retinopathy using tirzepatide should be monitored closely and treated according to clinical guidelines. There is no experience with tirzepatide 2.4 mg in patients with type 2 diabetes with uncontrolled or potentially unstable diabetic retinopathy.
This medicine contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially 'sodium-free'.
Interaction with other medicinal products and other forms of interaction
As with other GIP/GLP-1 receptor agonists, tirzepatide may delay gastric emptying and could potentially influence the absorption of concomitantly administered oral medicinal products. No clinically relevant effect on the rate of gastric emptying was observed with tirzepatide 2.4 mg. In clinical pharmacology trials assessing the effect of tirzepatide 1.0 mg on the absorption of co-administered oral medications at steady state, no clinically relevant drug-drug interactions with tirzepatide were observed based on the evaluated medications. Therefore, no dose adjustment is required when co-administered with tirzepatide.
Oral contraceptives
Tirzepatide is not anticipated to decrease the effectiveness of oral contraceptives as tirzepatide did not change the overall exposure of ethinylestradiol and levonorgestrel to a clinically relevant degree when an oral contraceptive combination medicinal product (0.03 mg ethinylestradiol/0.15 mg levonorgestrel) was co-administered with tirzepatide. Exposure to ethinylestradiol was not affected; an increase of 20% was observed for levonorgestrel exposure at a steady state. Cmax was not affected by any of the compounds.
Mounjaro Delivery — Step by Step
Note: Next Day Delivery is counted from the time your prescription is approved by our prescriber, not from when you place your order.
Online Questionnaire
Complete our secure medical questionnaire so our prescribers can confirm Mounjaro is safe and suitable for you.
Order & Payment
Place your order and pay — payment is held securely while the prescriber reviews your case.
If it's not suitable you will receive a full refund.Upload Images
Upload BMI images, a copy of your ID, and any additional evidence (if requested).
Check your spam/junk folder to avoid delays.Prescriber Review
A qualified clinician reviews your questionnaire and images. Respond promptly to any follow-up requests to avoid delays.
Approval & Dispatch
Once approved, we aim to dispatch for next-day delivery. Orders approved after the cut-off will be sent the next working day.
We take every step to maintain cold chain conditions (2°C – 8°C) during storage and transport of your Mounjaro® KwikPen, as this provides a stable environment for the medicine.
Once your Pen Arrives:
- We recommend placing it in your refrigerator (2°C – 8°C) until you are ready to use it.
- Protect it from light by keeping it in its original carton.
- Do not freeze — if frozen, the pen should not be used.
If your pen arrives at room temperature (up to 30°C), you can still use it.
- Mounjaro can be kept at temperatures up to 30°C for a maximum of 30 days.
- Your 4 doses will take 21 days to complete based on a 7 day injection cycle so there is ample time to complete your course.
- Store away from direct sunlight and heat.
- Always attach the pen cap before storage and never store with a needle attached.